

This website is a third-party knowledge base for researchers and nerds.
It is not intended for the general public and makes no claim to completeness, accuracy or being up-to-date. It can in no way replace a consultation with qualified medical personnel.
Consult your physician concerning any questions you may have concerning Pluslife test results.
Please note the following:
This website is not affiliated with Pluslife or Altruan.
The Pluslife test device is not for sale in the US and has not been approved by the US FDA.
The authors do not assume any liability for timeliness, correctness, completeness or quality of the provided information. Liability claims against the authors due to material or immaterial damage caused by the use or disuse of the provided information and / or by the use of incorrect and incomplete information are generally excluded. We do not assume any liability for the contents of external links, although we carefully review their contents. The operators of linked pages are exclusively liable for the contents of their pages.
Pluslife is a nucleic acid amplification test for the detection of SARS-CoV-2 RNA, similar to devices like Lucira or Metrix.
The tests are intended for professional users and only work correctly when used exactly according to the instructions for use. Carefully read the instructions before using the test, and take care to avoid air bubbles.
This website reviews the Pluslife test and helps users with common problems and questions. It is not affiliated with Pluslife or Altruan.
This document refers to the Pluslife SARS-CoV-2 test, unless stated otherwise. Pluslife offers a variety of other tests, which may have different characteristics. Make sure to read the instructions for use of the specific test you are using.
The manufacturer specifies a 95% LOD ("Limit of Detection") of 400 copies/mL for its SARS-CoV-2 test. The LOD is the lower limit of sensitivity: a sample containing 400 viral RNA copies per mL was correctly detected as positive in 19 of 20 samples when tested by the manufacturer.
This is an excellent result and comparable to lab PCR tests, which typically have LODs between 50 and 1000 copies/mL, depending on the quality of the assay.
For comparison: a rapid antigen test requires millions to tens of millions of RNA copies/mL, multiple orders of magnitude worse! Here is an attempt to represent these huge quantities as cube volumes:
Compared to conventional antigen rapid tests, which rely on direct detection of the pathogen, molecular tests like Pluslife rely on exponential amplification of genetic material. They can detect pathogens at lower levels and therefore earlier during the exponential amplification process, often before viral load is high enough for transmission.
Molecular tests such as Pluslife are very sensitive. However, keep in mind that - like any other method of relative risk reduction - they cannot guarantee 100% safety, and the absolute, effective risk greatly depends on your specific circumstances and the probability of someone being infected in the first place ("pre-test probability"). Which level of risk is acceptable or not is an individual decision and there are no "one size fits all" approaches to risk mitigation.
Unfortunately, Pluslife is not for sale in the U.S. It has no FDA approval, and would be subject to extremely high China origin tariffs even when shipped from Germany. Given the circumstances, it is unlikely that Pluslife will pursue FDA approval in the near future.
Be careful with any "workarounds"—while they may work today, they could stop working at any time, and you'll be left with an expensive paperweight once your supply of tests runs out.
Similar considerations apply for any other countries where Pluslife has no regulatory approval. Pluslife is approved in the EU, and possibly Hong Kong and New Zealand (we're not 100% sure).
Start the test immediately after the card is inserted into the device. The biochemical reaction starts as soon as the card is inside the device, regardless of whether the button has been pressed. If you wait too long, you will get an invalid or false result. This is very important.
The device should not be placed in the sun or exposed to direct sunlight during the test. Bright light interferes with the sensitive optical sensors inside the device. Do not open the lid during tests.
Avoid extreme heat or cold while operating the device. Condensation inside the device destroys it.
We recommend using a power bank (such as from Anker) instead of the supplied power supply unit.
Make sure to wait for ~15 seconds after screwing on the lid before pressing the top of the lid. This ensures that the liquid has enough time to fill the reaction chambers.
Once removed from the packaging, avoid exposing the test card and the buffer tube to bright light for extended periods. Molecular probes are very sensitive to light.
Do not forget to switch off or unplug the device - its heater will happily continue to heat. There is no automatic shutdown.
Do not overdo it when shaking the buffer tube and the test cards, otherwise foam and air bubbles will form, which can lead to invalid results. After thoroughly "squeezing" the swab in the tube, it is sufficient to invert and gently flick the tube several times to mix reagents.
If visible foam or air bubbles have formed, let the tube briefly rest while standing upright before transferring the fluid into the test card to prevent bubbles from entering it.
Process the sample material as quickly as possible: the viral RNA dissolved in the lysis buffer degrades relatively quickly. If necessary, store the unprocessed sample in the refrigerator or - ideally - freezer if immediate processing is not possible. Always insert the test card immediately into the instrument after transferring the sample and never store it.
Keep kit components out of the reach of children.
Make sure that the lid of the test card is properly secured.
Check test cards for damage or chips before using it.
Keep electronic devices which generate strong electromagnetic fields - such as mobile phones or radios - away from the device while a test is running.
Do not touch the surface of the test chambers on the cards. Oils on the fingers can impair the optical readout of the test chambers.
Wear disposable gloves and goggles. The buffer liquid is corrosive. If liquid does get on the skin, wash immediately with soap. In case of contact with eyes, rinse with clean water immediately and seek medical advice.
Put used test cards (esp. positive ones) into one of the safety waste bags that come with the kit and dispose of them immediately. Refer to your local regulations for disposal.
Do not re-test a card which had an invalid result, but try again with a new test card.
Remove the test cards promptly after the test, otherwise some of the liquid inside will slowly evaporate despite the lid. This applies especially to positive tests.
Nucleic acidic amplification tests like Pluslife are based on the exponential amplification of target gene sequences. The viral RNA is first converted to DNA (reverse transcription) and the target sequences - if present - are then amplified using a polymerase enzyme. A few RNA copies become billions of DNA copies, which can be easily detected using an optical method.
However, this also means that the test cards contain very high quantities of these DNA snippets ("amplicons") after the test. Although these are harmless to humans and the environment and are in no way infectious, they can contaminate the environment if handled incorrectly.
The procedure is very sensitive and even the smallest quantities that escape are sufficient to contaminate subsequent reactions or even people, which then leads to false positive results. Pluslife tests would then become useless in contaminated rooms or for persons exposed to environmental contamination.
DNA is very robust, cannot be removed with most disinfectants and degrades very slowly (unlike viral RNA). It can remain detectable in indoor air and dust for years. Contamination should therefore be avoided at all costs.
Never open the test card after the reaction and remove cards from the device immediately after testing. Dispose of them in the bags provided.
Handle positive tests with particular care, always remove the test cards with protective gloves and then change the protective gloves.
If the test card does leak after a positive result, false positive results must be expected in the future. If possible, dispose of or decontaminate all objects that have come into contact with the liquid using disposable gloves.
If contamination is suspected, the environment and the test device can be swabbed with a damp swab and then tested normally without a human sample. If this control is positive, although no one in the environment is ill, there may be contamination.
DNA contamination is very rare and we are not aware of any cases outside of laboratories. If you suspect contamination due to a positive environmental control, we will be happy to help you with decontamination - please contact us by email.
Pluslife tests are very specific and false positive results are rare.
However, it's possible to get false positive results from improper usage of the device - such as introducing air bubbles, opening the lid during amplification, inserting the test card after the test is already running, or removing and re-inserting the card during a test.
Air bubbles can move within the test card due to the heat. Condensation can form within the air bubble, which can cause a sudden increase in the optical signal, which the device then incorrectly evaluates as a positive result.

With our app such an abrupt increase is easy to recognize by an expert.
If false positive results are suspected, the test should be repeated entirely, including taking a new sample and, if possible, using a different batch of the test kit. If the result is negative on retesting, it is a false positive result. If the result is positive again, a positive result should be assumed.
In addition to the actual Covid target genes, the test cards also contain an internal control that tests for a gene sequence present in each sample and is therefore always positive. It tests for the presence of the human housekeeping gene β-Actin.
If the internal control fails to amplify or the signal isn't strong enough, the result will be invalid.
There are a number of possible reasons for invalid tests:
It may be possible for a defective device or bad power supply to cause invalid results. If you get consistent invalid tests and ruled out all other possibilities, try using the original power supply and cable (see above regarding power supplies). If that doesn't help, contact your seller to get your device replaced. If either of these solves the problem, please let us know (email address is at the bottom of the page).
The test kits should be stored in a cool and dry place between 2°–28° C.
The manufacturer has stated that the test kits have remained stable in experiments between -20–55 °C, so temperature fluctuations during transportation are harmless.
We are not aware of any studies that could conclusively answer the question. It is a question of individual risk assessment and pre-test probability (overall risk).
While there is no "golden rule", many users have successfully avoided household infections with a re-test window of ~12 to – at most – 24 hours.
A negative molecular test is a very strong indication that the person was not infectious at the time of the test. Within the incubation period since the last higher-risk contact, this can of course change and the test must be repeated regularly within the possible incubation period.
If the risk is very high, it makes sense to repeat the tests more frequently than if the risk is low.
The more sensitive a test, the earlier it can detect the virus at the start of the exponential growth phase when viral loads are still very low. This implies a greater "safety buffer" than less sensitive tests: the period during which the virus can already be detected but the person is not yet infectious. While this safety buffer is essentially zero with normal rapid tests, a molecular test such as Pluslife offers a certain degree of safety.
The manufacturer told us that both nasal and throat swabs will work on SARS-Cov-2 tests, and mentioned that the reason the package insert does not mention throat swabs is due to the accelerated certification process at the beginning of the pandemic and is no longer accurate.
Covid tends to show up in throat samples much earlier than in nasal swabs, which is why many Pluslife users perform a throat swab or combined nasal and throat swab to detect infection as early as possible.
In the Imperial College Human Challenge study, almost all patients had detectable virus in their throats 1-2 days earlier than in their noses. We marked the time delta with red bars:
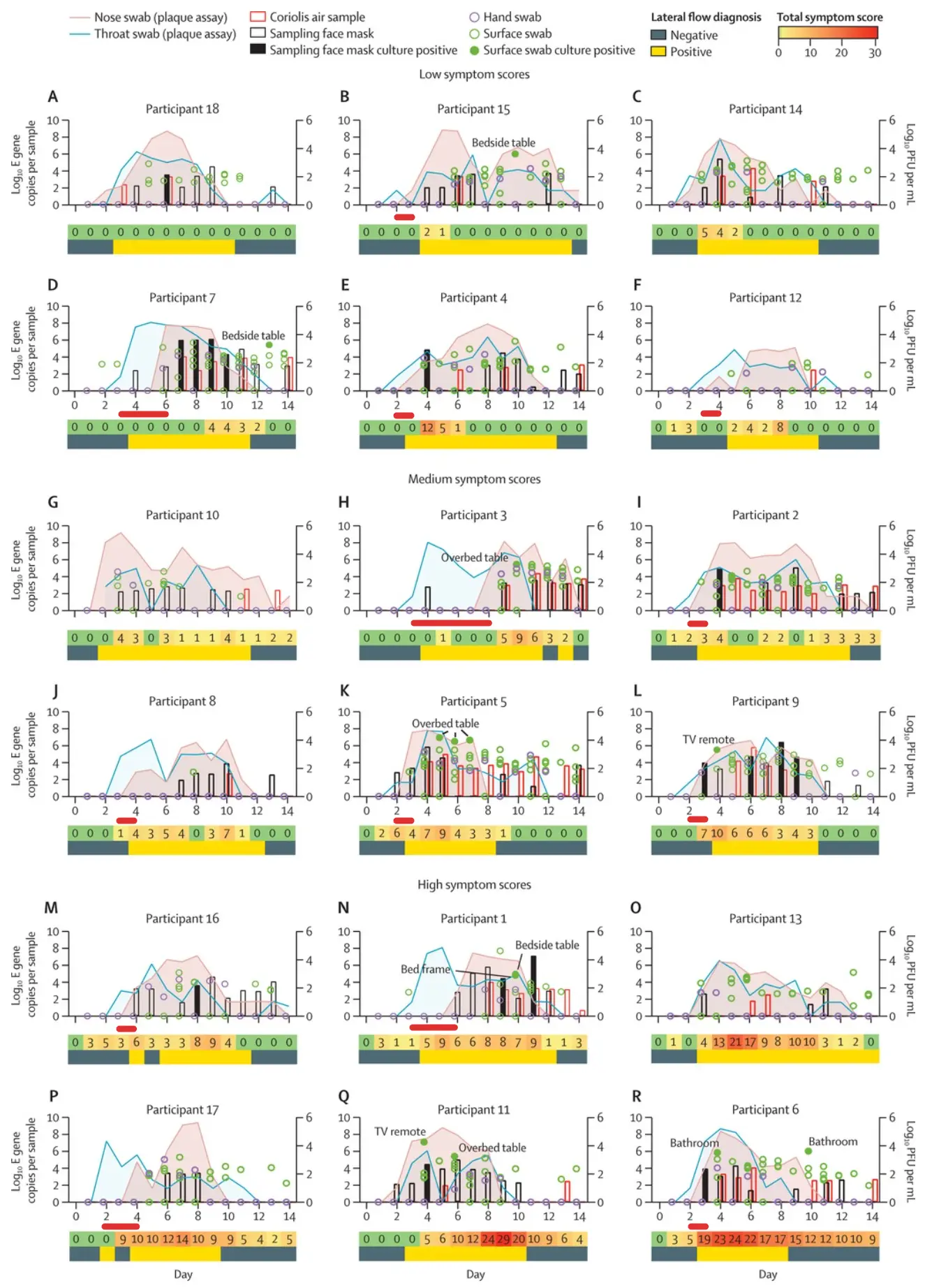
While there is some uncertainty due to the lack of clinical studies with throat swabs, the data suggests that a throat swab or combined nasal and throat swab may be best for early detection.
A good time to swab is after getting up before breakfast. Nasopharyngeal swabs also work, but require special training and are not necessary for an accurate test result.
When doing a throat swab, it is important not to eat or drink anything at least 30-60 minutes beforehand to avoid interference. Avoid acidic foods – the test is very sensitive to pH changes.
Pluslife has not been validated for pool testing, all studies were carried out with individual samples.
Many Pluslife users have had good experiences with small pools (3-4 people maximum). However, the risk of inhibitors and invalid result or reduced sensitivity increases with each additional person. The liquid must also not become too thick, otherwise it can no longer flow through the small capillaries on the test card.
For pools, individual swabs are extracted one after the other in the buffer solution and then tested normally. If the overall result is positive, swab again and test individually.
It is important to consider in advance how a positive pool would be handled. In the event of a positive test, it takes a long time to resolve a large pool. If possible, consider using multiple devices instead of increasing pool size.
As far as we could tell, samples should be processed immediately.
Under no circumstances should the sample be stored in the Pluslife buffer solution. The buffer solution is aggressive and the sample should be processed within a few minutes after extraction, otherwise the RNA will decompose and will no longer be detectable after some time.
If there is no other option, the swab should be stored in a fridge in a plastic bag or in an empty protective tube to protect it from drying out. Freezing is best, but make sure to fully thaw the sample before processing.
Check the label on the tubes - it will indicate the type of buffer (such as Nucleic acid release agent 01). If it's the same buffer, it can be used interchangeably. For instance, SARS-Cov-2 tests and FluA/FluB/RSV tests use the same buffer (01), but StrepA tests do not (02). Always re-check the label on your specific lots to make sure they are compatible and make sure the buffer isn't expired.
If the buffers are identical, you can run different tests in parallel with just one sample. There is enough liquid in the tube for two tests. However, we recommend using multiple devices to run both tests at the same time to avoid exposing the sample to the buffer solution for too long. If you have only one device, use a fresh sample and a new tube for the second test.
It occasionally happens that the liquid in the test tubes gels. Various factors can contribute to increased sample buffer viscosity.
The cells introduced into the sample are lysed in the liquid. During lysis, DNA and RNA contained in cell nuclei and cytoplasm are released. These molecules are long-chain and can be very viscous in high concentrations, which makes the solution more viscous. This occurs particularly when too much material is introduced.
It is thought that small amounts of blood, which contribute to the viscosity through the introduction of clotting factors such as fibrinogen, may also play a role. Users have reported that it can be helpful to unscrew the cap if the liquid would otherwise no longer fit through the filter.
The test should be repeated with a new swab if the fluid gels. It may be helpful to avoid a nasal swab and just swab the throat instead.
Yes, Pluslife sells a variety of different test kits. While this document focuses on SARS-Cov-2, tests for influenza A/B, RSV, Strep A and more are available. More details can be found on the manufacturer's website.
For tests other than Covid, it is necessary to use the device with an app – rather than by pressing the button on the device – so that the test is carried out correctly and the individual results can be displayed for combination tests.
The interaction with nasal sprays such as VirX and Bioblock is unknown.
When using nasal sprays, an interval of at least several hours should be maintained or a nasal swab should be avoided altogether.
Current studies not provide a clear answer to this question.
Due to their sensitivity, molecular tests such as PCR or Pluslife are of dubious utility for determining the end of an infection. They can measure the presence of (very small amounts of) viral RNA, but can only provide a rough indication of the viral load and no indication of contagiousness.
A Pluslife test can be positive even if the viral load is very low and there is no longer any risk of infection (days to weeks). The course of the disease is very individual. The decision should therefore be made according to a clinical evaluation and not purely on the basis of the test result.
Yes, with combination tests there are fewer channels available per test, which reduces sensitivity. The Covid/Flu combination tests, for example, have an LOD of 1000 copies instead of 400. For early detection of Covid, we recommend individual tests.
For clinical diagnosis, the difference is usually negligible.
No, the manufacturer specifically advises against it. After thoroughly "squeezing" the swab, the test should be started immediately (within at most five minutes).
Yes, the manufacturer states that if you happen to have one (such as in a lab), you can use it for mixing the buffer tubes as well as for the test cards. Do it briefly and gently. Vortexing too much can destroy the reagents.
It is not necessary to use a vortex mixer, but it can simplify the process considerably and improve the reproducibility of your results, especially for high throughput testing.
This is normal and harmless - the pressure exerted on the test card when it is screwed shut is usually enough to force the liquid into the chambers. Nevertheless, always press in the lid, even if it appears unnecessary.
Tiny air bubbles are normal and harmless. Large air bubbles can disturb the test procedure - if in doubt, discard the card, use a new test card and be more careful when filling it.
After testing, there are always small bubbles in the chambers, this is also normal.
This is normal. The chambers already fill before the lid is pressed in. As long as the liquid level was between the lines directly after filling the card, you're good! Do not add any extra fluid afterwards.
Unfortunately, the lines can be hard to see. Good overhead lighting helps. You can also mark the lines with a thin permanent marker ahead of time.
We asked Pluslife whether they could add markings in the factory, but unfortunately, it would make the tests more expensive since extra machines are required.
Looks can be deceiving at a first glance. The chambers can look empty when they are filled entirely.
If there is really no liquid in the chambers, the sample was too thick. Resample and, in the case of a pool, reduce the number of pool participants.
The powder only dissolves completely during the test.
The breakage of the balls does not affect the test results. As long as the test card is within the validity period, and the appearance of it is normal, the ball's shape will not affect the test.
Yes.The manufacturer recommends transporting it in the original packaging to avoid damage from shocks.
The device is a solid-state device. It contains neither moving parts nor any transport safeties.
Nevertheless, the electronics are not indestructible. Strong shocks, vibrations, extreme heat or cold, condensation and similar mischief can damage the optics or the circuit boards. When transporting, pack it well and avoid exposing it to any harsh environments.
The device does not switch off automatically, the result would - in theory - still be readable days later.
However, the test card should be removed as soon as possible after the test, as the heater does not turn off after the test and some liquid will slowly evaporate. This is especially important for positive tests due to the risk of DNA contamination (see above).
The exact chemical composition of the kit is not known. The manufacturer states that the buffer fluid is corrosive and contact with the skin should be avoided, and while brief contact is harmless, strongly recommends immediately washing off any spillage with water and soap.
We recommend treating the kit like you would treat a strong household cleaner that may be toxic. Use protective gloves and goggles, keep away from children and pets.
The manufacturer strongly recommends not to use expired test cards.
We have also carried out our own tests and can confirm that expired tests no longer work properly and can even be false negative. The buffer solution degrades over time and becomes more acidic.
The fresher the test kit, the better it works. We recommend against stockpiling more than a six-month supply. Fresh batches are constantly being produced, it is better to order more frequently.
SARS-CoV-2 tests expire 12 months from the manufacturing date. When you order SARS-CoV-2 tests from Altruan, you usually receive a fresh batch - they're in high demand and don't stay in the warehouse for long.
Other tests and tests from distributors other than Altruan may have shorter expiration dates when you receive them.
If the reaction does not work at all because the enzymes are no longer active enough or the sample contains interfering substances (inhibitors), the internal control would not respond and the instrument would report an invalid test. The internal control therefore guarantees, to a some extent, that the test cards are still OK and will detect high viral loads.
However, it might be possible for the enzymes to degrade - for example, due to incorrect storage - but without failing entirely. In this case, the (highly concentrated) internal control would still work, but sensitivity at very low viral loads would be reduced. We don't know whether this is something that can happen to Pluslife assays. Overall, it is safe to say that kits which aren't expired and were stored appropriately will have preserved their original sensitivity.
Pluslife recommends using the device only with the original (OEM) power adapter and cable. Use an adapter if necessary.

If you experience any issues with the device and aren't using the original adapter, please first try using the OEM adapter to see if that solves the problem before contacting Altruan, Pluslife or us.
Pluslife is a sensitive lab device and as such, it requires a high quality power supply. It needs good voltage stability under load (noise/ripple) and must be able to supply 3A/15W on a USB-A port. Many commercially available power supplies or powerbanks do not meet these requirements.
Using a non-OEM power supply can cause device malfunctions, including disconnections and invalid tests.
The device can, in theory, be powered by any high-quality power supply which can supply stable, low-noise power at 3A at 5V.
In particular, powerbanks are useful for testing "on the go" or when there is strong interference which causes "jumpy" readings.
Refer to this great article by analogist.net regarding potential replacements with good noise/ripple characteristics.
Use a non-original power supply at your own risk. If you experience any issues, first try using the original power supply (with an adapter, if necessary).
This means that the heater has not reached its target temperature. This may be due to a defective power supply unit, inferior cable or even a defective device.
See above regarding the correct power supply to use.
Early versions of the Pluslife Minidock often have problems with defective heaters. We recommend that you contact whoever you bought it from.
Pluslife recommends using only the original cable, which has the correct length and current rating. Contact your seller for a replacement cable.
The cable is not a normal USB cable, but "USB to round plug". Such cables are commercially available, but not too common.
The size of the round plug (5.5 x 2.1 mm, male, DC 5521) and the polarity - minus outside, plus inside - are important. The length of the cable MUST be max. 1.5m with rating for min. 3A/15W. Otherwise, the voltage drop might cause device malfunctions.
Some materials can interfere with the reaction or have the wrong elution volume, which is why the manufacturer recommends using only Copan FLOQswab 502C swabs, which have been tested extensively.
They're available from Altruan (ignore the incorrect picture). Many local medical suppliers have them too, it's a very common brand and widely used for diagnostics.
Thinner FLOQswab swabs for nasopharyngeal swabs are also available. In Germany, they're sold by Praxisdienst. Most local suppliers should have them. Many users have had good experiences with the thin swabs for pool tests to avoid collecting too much material. Please note: Neither pool tests nor the use of thin swabs have been tested by the manufacturer.
The "normal" thick swabs are also available with a protective tube, which can be useful.
If it is not possible to use the original swabs, other swabs made of flocked nylon might also work - however, the manufacturer has not tested these and it's impossible to know which brands are problematic. Pluslife's release agent is unusually aggressive and Pluslife mentioned that some brands had issues where the glue would dissolve. Cotton, calcium alginate and coiled (instead of flocked) swabs are generally unsuitable.
In a LAMP reaction, DNA is amplified until the strength of the optical signal exceeds a certain threshold, similar to the cycle threshold (Ct) value in PCR.
The device reports the positive result immediately after the threshold was exceeded. However, as there is no such clear indicator for a negative result, the device simply waits and then reports a negative result as long as the internal control has worked and none of the target reactions are positive.
On average, it takes about 10-15 minutes for a positive sample to be reported. Always wait for the entire duration of the test: a weakly positive result can take up to 30 minutes to be reported.
Most Covid tests detect so-called "conserved epitopes", i.e. parts of the virus that do not mutate frequently. Therefore, new variants rarely cause false-negative results.
When Omicron appeared, there were significant mutations on the N protein, and some single-target antigen tests stopped working. Molecular tests such as RT-qPCR or Pluslife were not affected.
Molecular tests usually target multiple targets, so if one of the targets mutates, the other target(s) will still be detected. All targets would have to mutate at the same time to cause a false negative result.
Pluslife targets both N and ORF1ab gene sequences, and is therefore considered robust against mutations. The manufacturer most recently declared on 08/05/2025 that Pluslife's SARS-CoV-2 tests recognize current Omicron variants, including LP.8.1 (a derivative of the JN.1 and XEC variants) and NB.1.8.1 ("Nimbus").
No, you can only power the device via the round plug. The USB-C port is for data only.
Unplugging works just fine.
If, after a positive rapid antigen test, a follow-up Pluslife test is negative, we recommend repeating both tests several hours apart to exclude stochastic effects and errors. If available, use different lots of each test.
Pluslife is more sensitive and specific than rapid tests and a repeatably negative Pluslife result is therefore more conclusive than a positive rapid test.
We are personally aware of cases in which a symptomatic respiratory infection (of unknown cause) caused false-positive rapid tests in several individuals. In one case, RT-qPCR was performed in addition to Pluslife, which confirmed the negative Pluslife result.
The RKI has confirmed that both rhinoviruses and bacterial pathogens can lead to false positive results. There are isolated case reports in the literature (et al.). The exact frequency and mechanism of action are unknown, but it appears to be relatively common. Since the manufacturers of rapid tests exclude cross-reactions with other respiratory pathogens in vitro in quality assurance, we hypothesize that the mucosal immune response itself is causally involved and might interfere with the response.
In a series of experiments we have ourselves confirmed that many rapid tests are highly sensitive to acids and even small amounts can overcome the acid regulation of the buffer solution, cause nonspecific binding and thus false positive results due to physical effects. Especially in young children with reflux, this possibility should be considered in addition to other interfering substances.
The authors would like to learn more about this phenomenon and we will cover the cost of a respiratory multiplex PCR panel for false-positive rapid tests with negative Pluslife results. Our e-mail address is on the bottom of the page.
Rapid antigen tests are based on binding the antigens - i.e. viral proteins, not RNA - with special antibody complexes and thus making them visible to the naked eye.
The sample is first stabilized in a lysis buffer, which dissolves the proteins from the virus ("lysis") and raises or lowers the pH of the solution to a certain value ("buffer").
The buffer solution is then dispensed onto the test strip.
Viral antigens first bind to mobile antibodies at the beginning of the test strip, which are marked with a colored marker (usually Colloidal Gold).
These antigen-antibody-gold complexes then bind to another set of antibodies fixed in place on the test line:
This method is prone to false positive results, since antibodies can also bind to each other or to any other proteins under non-optimal conditions such as incorrect pH and other interfering factors. With modern RT-qPCR or LAMP (with probes), this is much less likely: both primer and sample must bind to specific target DNA sequences, which - unlike the protein interaction - cannot easily happen due to purely physical effects.
This also explains the big difference in sensitivity: there must be enough viral protein in the sample to be able to see the resulting complexes with the naked eye.
Rapid tests are therefore only effective when a high viral load is present in the sample. They are of limited use for early detection and prevention of infection.
It depends on whether the vaccine contains the pieces of the viral genome which the test targets.
For SARS-Cov-2, mRNA (Moderna, Pfizer), protein subunit vaccines (Novavax) or viral vector vaccines (AstraZeneca) encode only the spike protein, not the entire virus. Pluslife does not look for the Spike gene, which means that false positives for SARS-Cov-2 are impossible with these vaccines.
Live-attenuated vaccines contain "real" virus, which can cause false positives. For instance, many influenza vaccines are live-attenuated vaccines, which can result in positive FluA/B tests.
Yes. It's a convenient way to test the device and troubleshoot connectivity issues.
The service life is not an expiration date, but the expected lifetime of the device guaranteed by the manufacturer in a professional environment. In practice, it will most likely last much longer than the indicated minimum lifetime, it just won't be guaranteed or certified by the manufacturer.
According to Copan, the expiration date on swabs is related to the sterilization process. Sterilization is only guaranteed for a certain period. Expired swabs should work just fine.
For SARS-Cov-2, incubation times are relatively short. A good rule of thumb is to start testing after 24 hours and continue testing for 4-5 days during the possible incubation period.
The vet minidock appears to be technically identical to the human minidock. However, they're not approved or validated for human use.
Minidocks for other uses may be configured to run tests other than SARS-Cov-2 when you press the button on the device to start a test, rather than using the app. Make sure to always use the app to start your tests to ensure that the correct program is used to evaluate test results.
It depends - the test program doesn't just influence the way the results are interpreted, it also affects the temperature steps used by the device. Choosing the wrong test may lead to incorrect results.
SARS-Cov-2 and SARS-Cov-2/FluA/FluB combination tests use very similar temperatures, so it's probably okay if you mixed those up. However, you'll have to interpret results manually from the fluorescence curves. If you don't know how to do that, it's best to repeat the test with a fresh sample.
The tests have not been validated with saliva, so we don't have accurate data to answer this question.
The good thing about molecular tests is that they're highly sensitive, so they are more tolerant to suboptimal sampling techniques than antigen tests. Anecdotally, testing saliva works well and can be a good alternative when compliance is an issue (such as when testing little kids), especially if better compliance means testing can be done more frequently.
Pluslife has confirmed that the Flu tests work for H5N1. However, you won't know whether it is H5N1 or another influenza strain.
While this is a common problem with other molecular tests such as Lucira, false positives due to contamination or wrong pH are highly unlikely with Pluslife. We have never seen it happen.
The probe-based RHAM technology which Pluslife uses is highly specific and very resilient against non-specific amplification, unlike most LAMP assays. Both the primer and the probe have to bind to their target sequences for amplification to occur.
However, a very low pH value can inhibit the reaction and lower its sensitivity, potentially leading to false negatives at low viral loads.
Yes, blood will interfere with the test and should be avoided.
Studies with the manufacturer's involvement:
Independent studies:
Herrmann, Laura, Juliana Breuer, Tuan Ngo Duc, Nicole Thomé, Fatemeh Ghazaani, Sundrela Kamhieh-Milz, Julian Kamhieh-Milz, and Andreas Pfützner. “Comparison of the Diagnostic Accuracy of the Pluslife Mini Dock RHAM Technology with Abbott ID Now and Cepheid GenXpert: A Retrospective Evaluation Study.” Scientific Reports 14, no. 1 (2024): 13978 (doi.org/10.1038/s41598-024-64406-9)
Zhu, Dandan, Jing Huang, Bei Hu, Donglin Cao, Dingqiang Chen, Xinqiang Song, Jialing Chen, Hao Zhou, Aiqun Cen, and Tieying Hou. “Trial of the Pluslife SARS-CoV-2 Nucleic Acid Rapid Test Kit: Prospective Cohort Study.” JMIR Public Health and Surveillance 9 (2023): e48107 (doi.org/10.2196/48107).
Other sources:
The virus.sucks team has developed its own app for reading raw curves. It's a web app that works on almost any device with a Chrome or Edge browser.
Like the official applications, the app makes it possible to use test kits other than the Covid tests and to display the individual results.
Unlike the official apps, it also displays the raw amplification curve:
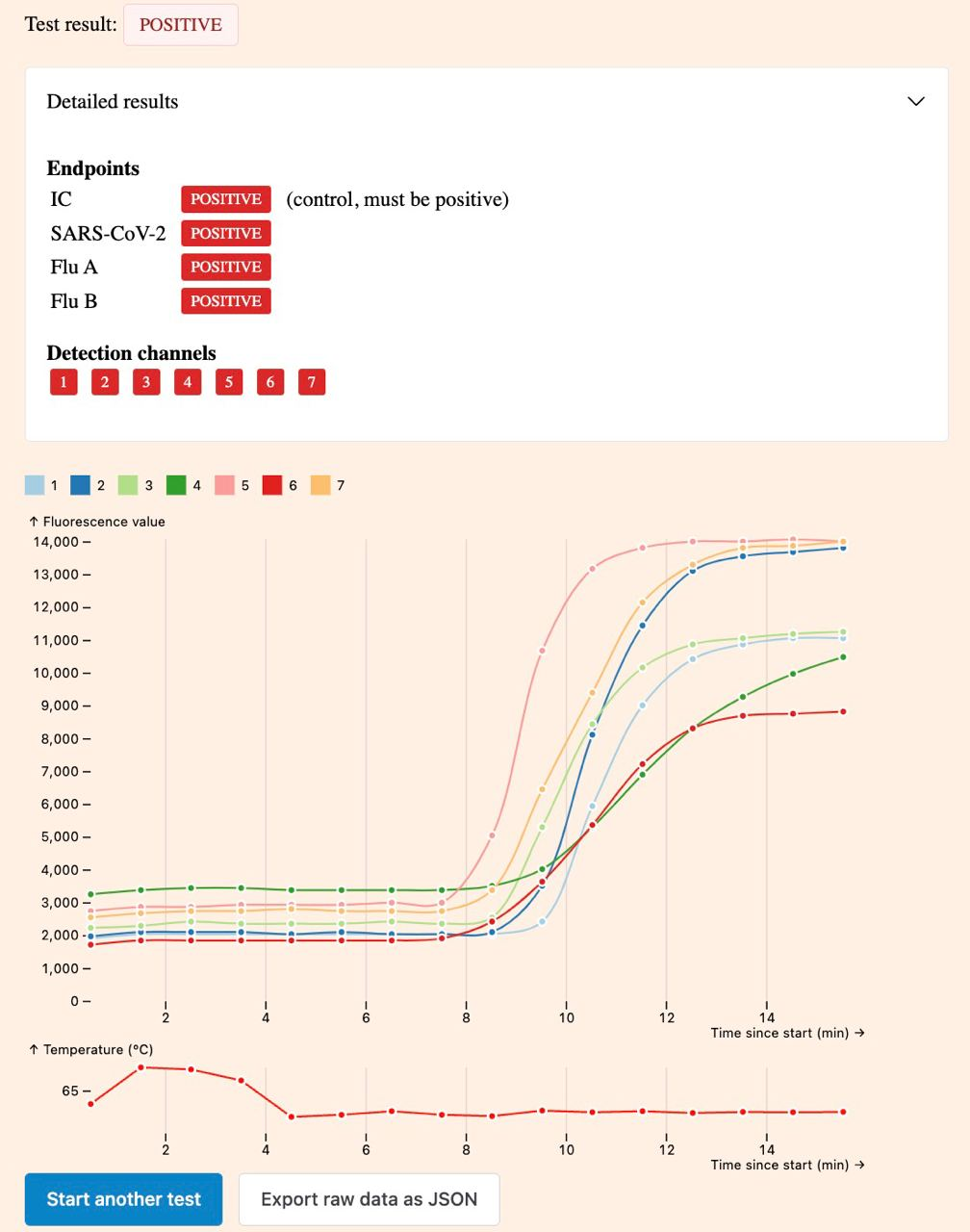
Manual interpretation of the amplification curve can enable a higher level of confidence when using the tests as well as higher sensitivity when used by an expert. There are some examples on Twitter, such as on air bubbles, very weak positive results and the dynamics of the reaction.
However, please keep in mind that the app has not undergone any official testing or approval and is not suitable for medical diagnosis, treatment, or any other medical purpose. It should not be relied upon to diagnose any condition or disease, or to initiate, continue, or modify any treatment regimen. Consult your physician concerning any questions you may have concerning Pluslife test results.
The app can be connected to the device via Bluetooth or USB-C and works on Windows, macOS, ChromeOS or Linux in Chrome, Edge, Vivaldi and other Chromium-based browsers.
Only Bluetooth works on smartphones. On iOS, the Bluefy browser is required.
Some very old Pluslife devices only support USB and no Bluetooth.
On Linux, start Chrome with --enable-features=WebBluetooth to enable Bluetooth.
For the serial console, make sure that the current user is allowed to read and write the device (e.g. /dev/ttyUSB0). With most distros, you can simply add the user to the dialout group and re-login.
Ubuntu or Ubuntu derivatives like Linux Mint may come with brltty by default, which can interfere with the connection. If you see a log entry like this:
[ 282.600777] usb 1-2: usbfs: interface 0 claimed by ch341 while 'brltty' sets config #1
...then try removing the brltty package.
If you installed Chrome/Chromium using a Flatpak or Snap package, it runs inside a sandbox, which does not allow access to USB or Bluetooth by default. Refer to the Flatpak or Snap documentation on how to allow access using snap connect or flatpak override. We recommend installing Chrome or Chromium using your distro's regular package manager instead.
Connect the app to the device and switch on the device as normal.
Then start the test not by pressing a button on the device, but after selecting the correct kit via the app. The test should be started at the same time as the test card is inserted into the device.
Keep the browser window and tab in the foreground during the test. Progress can be tracked in real time. If the test is negative, the control curve (channel 4) will increase exponentially after approx. 10 minutes, while the other curves will not change at all or only slightly. If the result is positive, several other curves will rise in addition to the control.
Manual evaluation of the curves requires experience and is a tool for experts.
Once the test has been completed, the final result is displayed - just like on the device. For combination tests, the results per pathogen are also displayed.
Pluslife also offers a range of applications for download, which can be connected to the device either via Bluetooth (smartphone apps) or USB-C (Windows).
Laboratories can use the Windows application to keep track of a large number of devices and assign the test results to individual samples.
We do not recommend using the smartphone apps, as they do not work reliably and are very difficult to install. Our own app offers a better alternative.
The manufacturer's app do not display the amplification curve.
For SARS-Cov-2 tests, at least two channels must be positive for the device to recognize the final result as positive.
When you use our app, you can see the raw data from the amplification, which means that it's sometimes possible for an expert user to identify positive results before the device does. This is the case when at least one of curves other than the internal control show clear signs of exponential increase (sigmoid shape, like the control reaction).
When there's clear signs of amplification on a single target channel, it indicates a weak positive result with low viral load, even when the device returns a negative result. For example, this is a positive result:
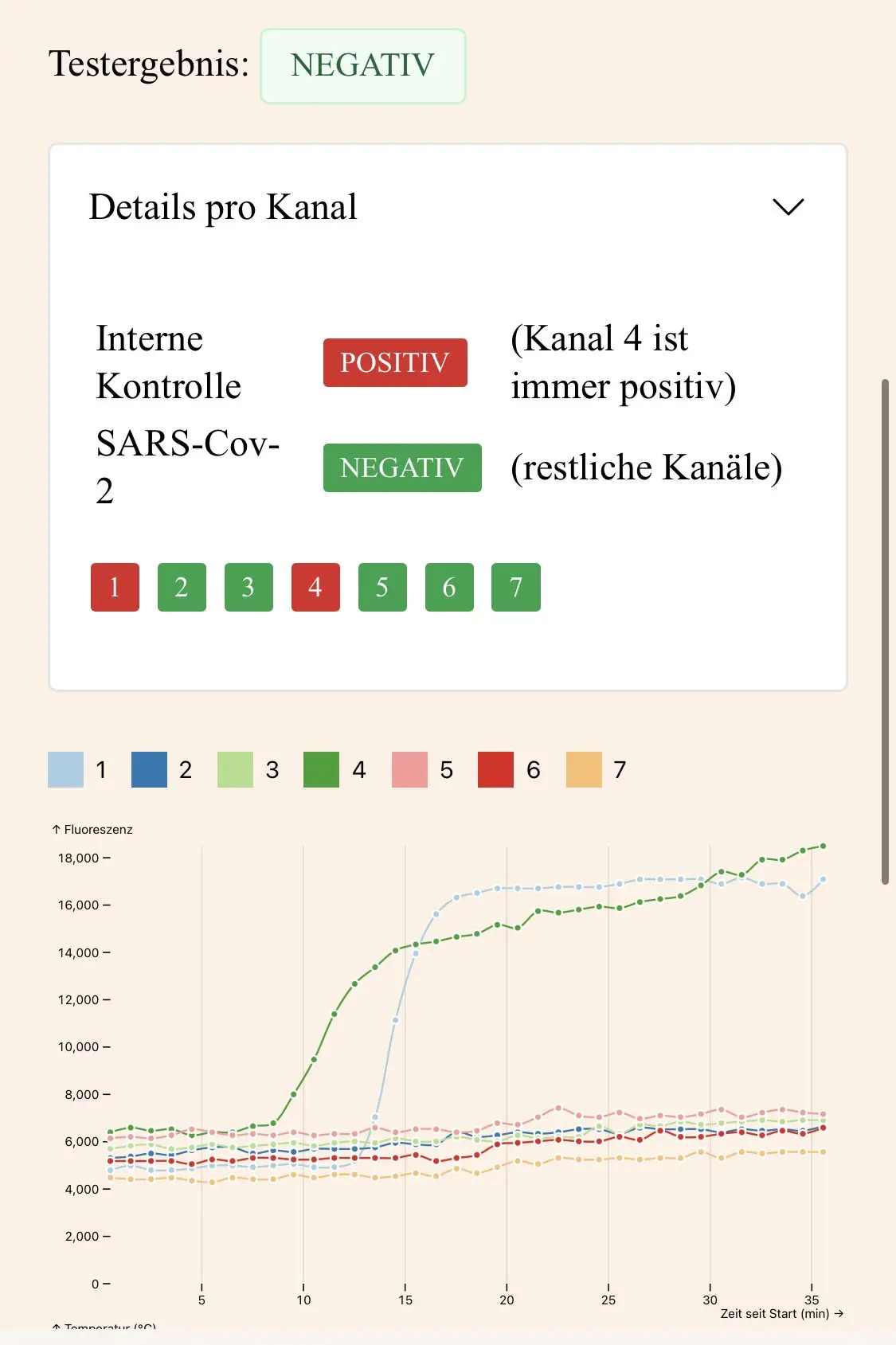
There are multiple possible reasons for weak positive results: An early warning about a fresh infection, viral fragments from a past infection (which can take weeks to fully clear), or even a failed infection which was successfully cleared by the immune system.
Rarely, weak positive results can also appear in the complete absence of an infection. We don't know why - it could be artifacts, low-level DNA or RNA contamination, or probe degradation/unspecific amplification.
A very slight, linear increase in some or all of the reaction channels is normal and does not indicate a positive result.
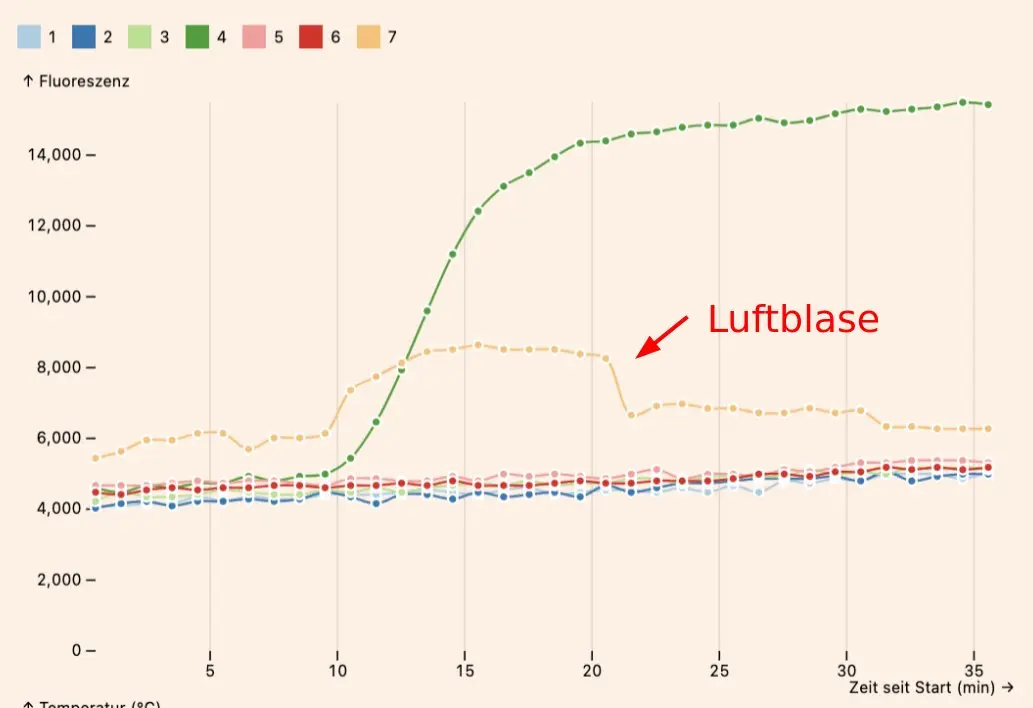
Air bubbles (incompletely filled reaction chambers) can cause invalid reaction curves which increase suddenly or jump around, due to optical refraction effects. These can even cause false positive results.
Make sure that the chambers are fully filled with liquid. Very small air bubbles tend to be OK, but large air bubbles will cause problems (see above for an example of an air bubble).
After screwing on the lid, make sure to wait ~15 seconds while some of liquid moves to the reaction chambers. Then gently press the top of the lid to force the rest of the liquid into the chambers. Doing it too quickly is the most common cause for air bubbles.
Fortunately, it is easy to distinguish an air bubble from a real amplification result. A true amplification result has a sigmoidal shape and will never increase or decrease suddenly:
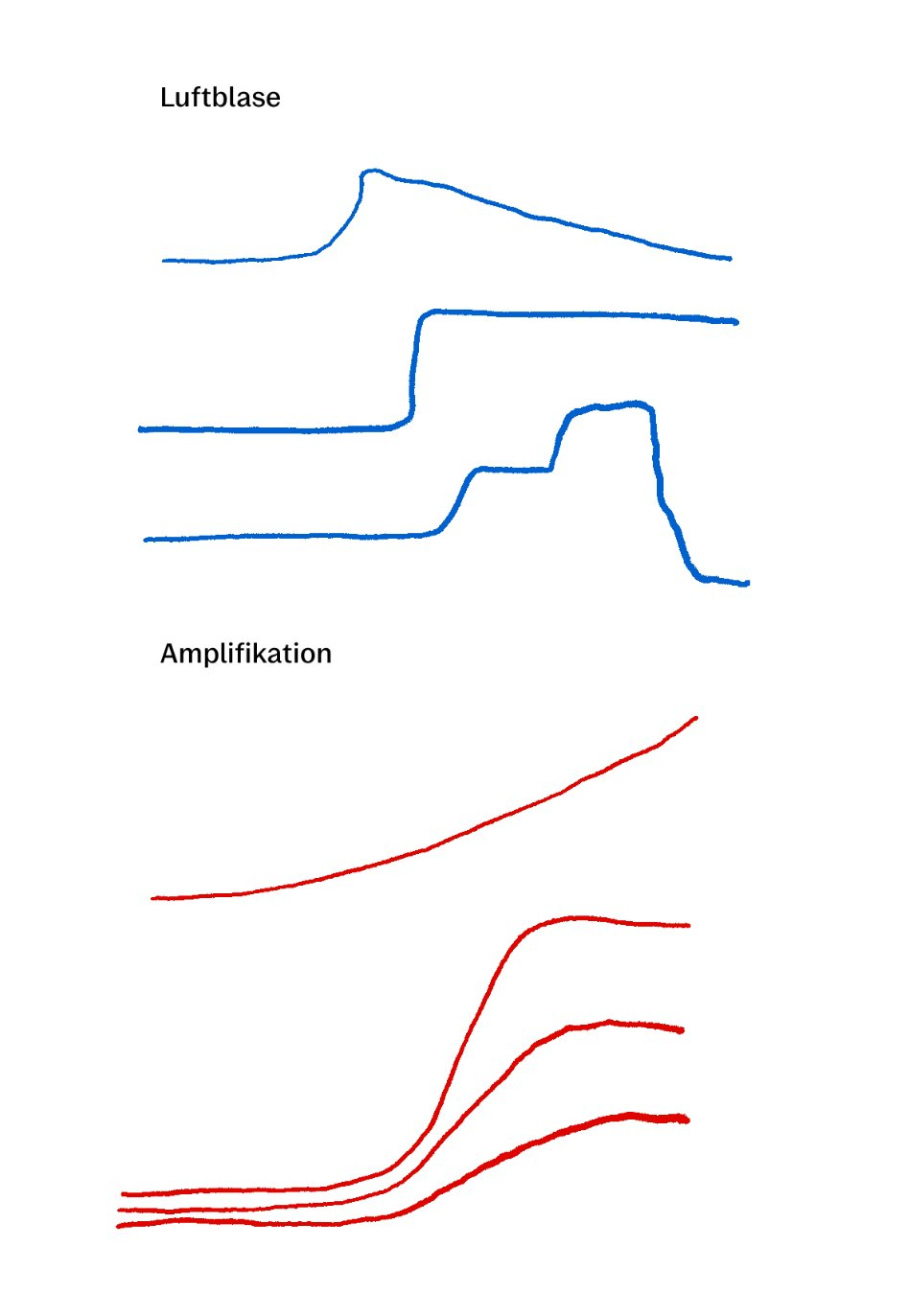
This is an air bubble:
Generally, no. Pluslife assays are optimized for qualitative (yes/no) detection rather than quantitative results.
In a clinical report from Israel, time-to-positive did not correlate with RT-qPCR Cq value over most of the relevant range. Only very low viral loads resulted in higher time-to-positive:
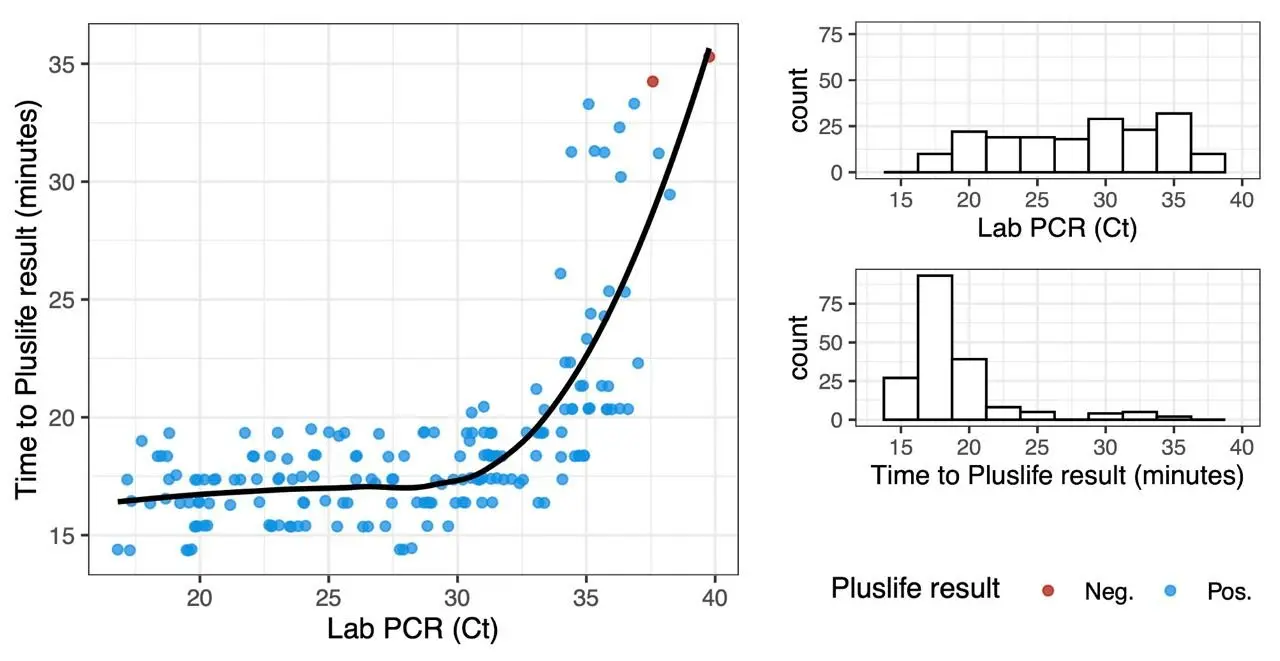
(data from the report, graphic our own)
Data from our own experiments confirmed this. In our experiments, time-to-positive was correlated to pH value much more strongly than viral copy number. Therefore, it is our opinion that Pluslife assays are not suitable for any kind of quantification.
There's a small exception: When only some, rather than all, of the target curves amplify (drop-out), the viral load is likely to be very low. However, that's not an exact science either. For reliable quantification, a quantitative RT-qPCR test is required.
| LED state | Meaning |
|---|---|
| Red blinking | Heating up |
| Blue steady | Ready to test |
| Blue blinking | Test is running |
| Red steady | Positive result |
| Green steady | Negative result |
| Red + green steady | Invalid result |
No. When a test is interrupted by a power outage or similar, the device will not remember the state of the test. Attempting to run the same test card again can lead to inaccurate results, even when the device displays a valid result, since the reaction occurs only once.
Unfortunately, the Bluetooth and USB connectivity to the Pluslife device is not always stable. This is a hardware issue, so there's not much we can do about it. Refer to our troubleshooting guide for tips on how to improve reliability.
When the app disconnects during a test, you can sometimes press "Reconnect" to re-establish the connection. If that doesn't work, you can restart the app and re-connect to the running test, but parts of the amplification graph will be missing.
Even if the app loses connection to the device, the test continues to run on the device and the result will be displayed on LEDs. You only lose the additional information from the app.
It always takes a few minutes for a real amplification reaction to start. If a curve starts going up immediately after the test starts (i.e. at minute zero), while the control reaction happens normally after a few minutes, it's an artifact and should be ignored.
It is particularly common in Flu, RSV and Strep tests.
If the control reaction is very flat (i.e. low reaction efficiency), it means that something has disturbed (inhibited) the reaction. The result is still valid, however, sensitivity might be reduced.
See above for potential causes. Repeat the test for optimal sensitivity.
Yes, this is possible. Open our Pluslife app in multiple browser windows - not tabs - such that all apps are visible on the screen. Then connect them to the devices one by one (you can use the serial number in expert mode to figure out which is which).
USB is more stable than Bluetooth.